ChemInform Abstract: Asymmetric Synthesis of (-)-(R)-Pyrrolam A Starting from (S)-Malic Acid.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
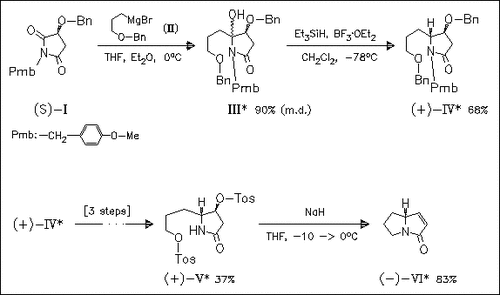
Key steps in the asymmetric synthesis of the title alkaloid (VI) are the highly regioselective addition of Grignard reagent (II) to the (S)-malic acid derived imide (I), followed by highly diastereoselective reduction of hydroxy derivative (III) to provide trans-pyrrolidinone (IV) in good yield. Suitable deprotection—protection sequence then affords the ditosylate (V), which in the presence of NaH undergoes a tandem tosic acid elimination—intramolecular N-substitution to give the title alkaloid (VI).