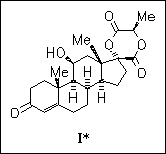
ChemInform Abstract: The 17-Spiro Lactide of Cortienic Acid: A Probe for Studying the Active Sites of Steroidal Receptors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The spiro lactide ring of title steroid (I) adopts half-chair conformation, with the two ester carbonyl groups aligned and directly opposing each other. The crystal packing diagram shows that the molecules are connected in a two-dimensional zigzag pattern by hydrogen bonds involving the 11-hydroxy and 3-keto groups.