ChemInform Abstract: A Single-Pot, Mild Conversion of β-Lactones to β-Lactams.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
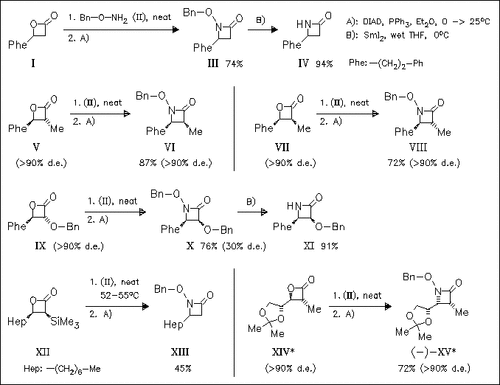
β-Lactones can be converted to β-lactams in two steps via N-benzoylhydroxamic acid derivatives, obtained by treatment of the starting lactones with hydroxylamine (II) without or with minimal solvent (Et2O), and subsequent intramolecular Mitsunobu reaction. This sequence proceeds with inversion of configuration. In the case of benzyloxy derivative (X), epimerization during the reaction leads to a decreased d.e. of product (XI). The reductive N—O bond cleavage is readily accomplished using SmI2, in the case of derivative (X) without concomitant cleavage of the α-benzyloxy group.