ChemInform Abstract: The Intramolecular Diels—Alder Reactions of Photochemically Generated trans-Cycloalkenones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
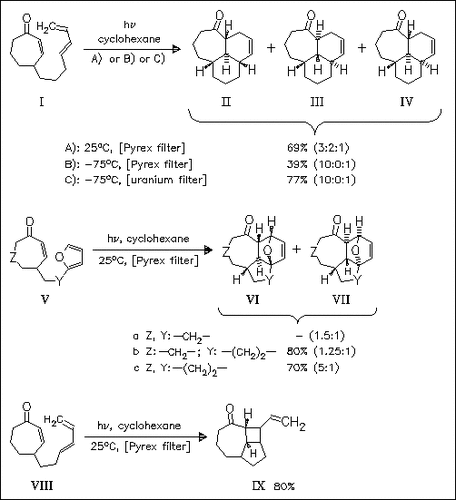
A novel rapid synthetic way to complex polycyclic structures is presented. Photoinduced isomerization of cycloalkenones like (I) and (V) affords highly reactive trans-cycloalkenones, which readily undergo intramolecular Diels—Alder reaction with the attached diene moiety. The corresponding cycloadducts are obtained as diastereomeric mixtures, but main diastereomer in each case is the all-trans-isomer [cf. (II), (VI)]. Irradiation of cycloheptenone (VIII), containing a two-carbon-atom spacer between ring and diene moiety, furnishes only 15% of a Diels—Alder adduct, in contrast, the [2 + 2]-cycloadduct (IX) is obtained. Its furyl analogue (Va), however, smoothly undergoes [4 + 2]-cycloaddition reaction to give the expected adducts (VIa) and (VIIa).