ChemInform Abstract: Ring-Opening Iodo- and Bromosilation of Cyclic Ethers by Treatment with Iodo- and Bromotrialkylsilane Equivalents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
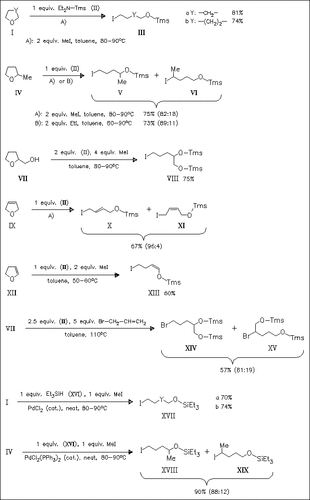
The ring-opening halosilation of five- and six-membered cyclic ethers using 1:2 mixtures of Et2NTms/alkyl halogenide (MeI, EtI, EtBr, AllylBr), or 1:1 mixtures of silane (Et3SiH, PhSi(Me)2H, Ph2Si(Me)H) and alkyl halogenide (MeI, AllylBr) in the presence of catalytic amount of palladium catalyst affords bifunctional halo(siloxy)alkanes, including halogenated silyl enol ethers.