ChemInform Abstract: The Pd(II)-Catalyzed and the Thermal Claisen Rearrangement of 2-Alkoxycarbonyl-Substituted Allyl Vinyl Ethers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
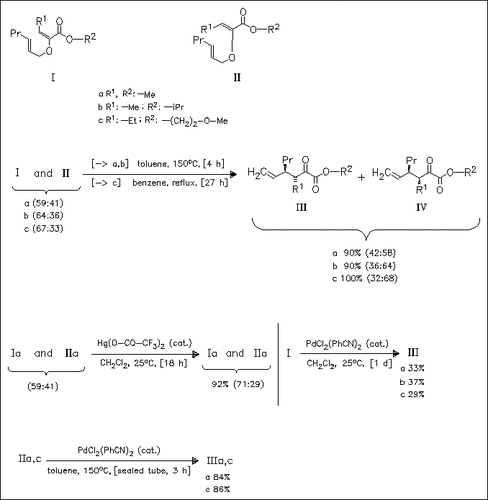
The thermal rearrangement of the vinyl ethers (I)/(II) gives syn/anti mixtures of the keto esters (III) and (IV). In the presence of a mercury catalyst, only double bond isomerization is observed. When a Pd catalyst is used, the (E,E) isomers (I) rearrange at 25°C to anti (III) (boatlike transition state), whereas at 150°C the (Z,E) isomers (II) give (III) (chairlike transition state).