ChemInform Abstract: The Photolytic and Hydrolytic Lability of Sisyl (Si(SiMe3)3) Ethers, an Alcohol Protecting Group.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
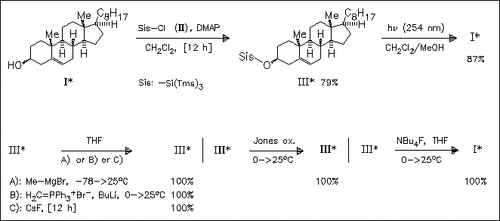
A detailed account of recently published studies on the scope of the sisyl protection group is presented. Sisyl ethers are found to be stable to less reactive organometallic compounds, phosphorylides, oxidation, and selected fluoride salts. Reaction with BuLi or LiAlH4 furnishes mixtures of deprotection and decomposition compounds. Clean deprotection is achieved by photolysis or using TBAF. Relative rates for acidic hydrolysis are determined. Following orders for the ease of hydrolysis are observed: RO-Si(Tms)3 > RO-Tbs (aq. AcOH) and RO-Tbs > RO-Si(Tms)3 (TosOH×H2O, CDCl3).