ChemInform Abstract: 1,3-Stereocontrol with Phosphine Oxides: Asymmetric Synthesis of all Four Diastereoisomers of a γ′-Benzyloxy β-Hydroxy Phosphine Oxide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
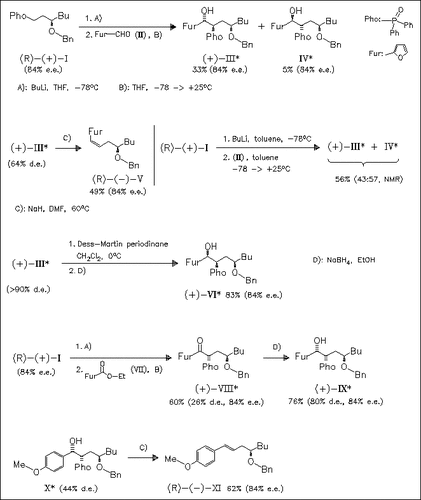
Several optically active phosphine oxides exhibit moderate levels of 1,3-asymmetric induction by lithiation and subsequent reaction with aldehydes or esters to give the title compounds. Complementary routes to all four diastereomeric alcohols (III), (IV), (VI), and (IX) are developed starting from the same phosphine oxide (I). The anti phosphine oxides (III) or (IV) are synthesized by addition of aldehyde (II) to the lithiated phosphine oxide. Depending on the solvent, THF or toluene, diastereomers (III) or (IV) are formed predominantly. Alternatively, β-keto phosphine oxides, generated either by oxidation of the alcohol (III) with Dess—Martin periodinane or by acylation of phosphine oxide (I) with ester (VII), are reduced with NaBH4 to give the syn isomers (VI) or (IX) with high selectivity. The success of the complementary routes hinges on the configurational instability of the lithiated phosphine oxides. The access to anti and syn diastereomeric series is possible because aldehydes and esters react preferentially with different isomers of a rapidly equilibrating mixture of lithiated phosphine oxides. The β-hydroxy phosphine oxides are shown to be valuable intermediates in the synthesis of optically active homoallylic alcohol derivatives like (V) and (XI).