ChemInform Abstract: A Mild Preparation of α-Halo-α,β-enones from Cyclic Enones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
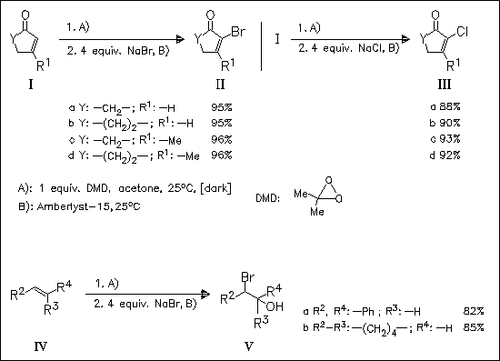
The selective transformation of cyclic enones (I) to α-halo-α,β-enones (II) and (III) is achieved in a simple one-pot procedure by oxidation of the parent enones (I) with dimethyldioxirane and subsequent treatment of the epoxide intermediates with alkali halides in the presence of Amberlyst. Olefins (IV) are converted to halohydrins (V) under these conditions.