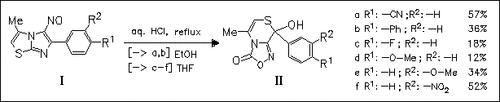
ChemInform Abstract: Ring-Ring Interconversions. Part 2. Effect of the Substituent on the Rearrangement of 6-Aryl-3-methyl-5-nitrosoimidazo[2,1-b][1,3]thiazoles into 8-Aryl-8-hydroxy-5-methyl-8H-[1,4]thiazino[3,4-c] [1,2,4]oxadiazol-3-ones. A Novel Class of Potential Antitumor Agents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Rearrangement of imidazothiazoles (I) into thiazinooxadiazoles (II) is largely substituent-dependent and leads with electron-repelling or -withdrawing substituents at the benzene ring to low or high yields, respectively. A multistep reaction mechanism is discussed.