ChemInform Abstract: Stereoselective Synthesis of Racemic and Optically Active E-Vinyl and E-Dienyl Sulfoxides via Wittig Reaction of α-Sulfinyl Phosphonium Ylides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
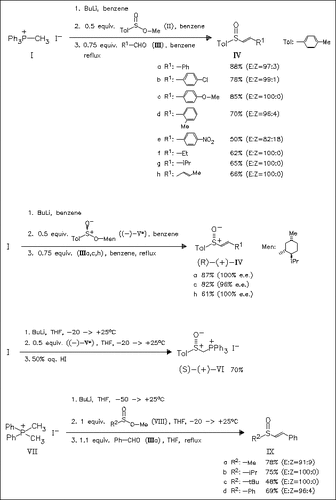
A new stereoselective method for the synthesis of the title sulfoxides is presented which is based on the Wittig reaction of intermediate α-sulfinyl phosphonium ylides with various aldehydes. The latter compounds are afforded from phosphonium monoylides or diylides (derived from phosphonium iodides (II) or (VII)) upon treatment with sulfinic acid esters. In most cases the target sulfoxides are afforded as pure E-isomers. Phosphonium iodide (VI) gives with (E)-cinnamaldehyde the corresponding enantiopure (E,E)-dienyl sulfoxide, but the Wittig reaction under phase-transfer conditions with benz- or formaldehyde gives only less satisfactory results in terms of (E)-stereoselectivity and optical purity.