ChemInform Abstract: Catalytic and Structural Features of Hydroxy and Methoxy Groups as Hemilabile Coordinating Ligands in Chiral (Diphosphane)rhodium(I) Hydrogenation Catalysts.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
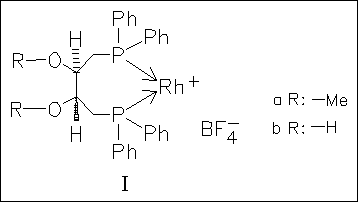
The catalytic activity of hydroxy- and methoxy-substituted diphosphane rhodium complexes in asymmetric hydrogenation of prochiral olefins, e.g. itaconic acid, is investigated in comparison to the unsubstituted parent complex. Introduction of the methoxy substituent in (Ia) reduces seriously the reaction rate and the hydroxy substituent still enhances the loss of reactivity but leads to significantly higher enantioselectivities (by ca. 35% e.e.) than the methoxy substituent.