ChemInform Abstract: Regiocontrolled Acylation of myo-Inositol Orthoformate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
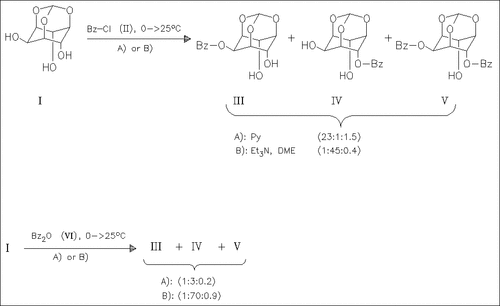
The acylation of myo-inositol orthoformate (I) is investigated. Using benzoyl chloride (II) as acylating agent and pyridine as solvent and base results in selective reaction at the equatorial hydroxyl. Benzoic anhydride in DMF/Et3N allows a highly regioselective acylation of the axial hydroxyl.