ChemInform Abstract: Asymmetric Allylation and Reduction on an Ephedrine-Derived Template: Stereoselective Synthesis of α-Hydroxy Acids and Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
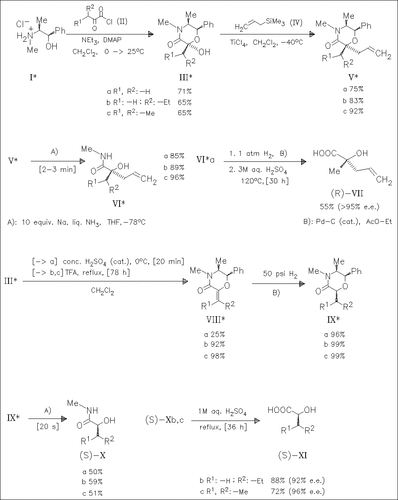
The asymm. synthesis of α-hydroxy acids is of active interest due to their utility as chiral building blocks. The high stereoselectivity in asymm. C—C and C—H bond construction with the chiral hemiacetals (III) derived from α-keto acids and (1R,2S)-ephedrine is demonstrated. The methodology described has been applied to the stereoselective synthesis of several α-hydroxy acids and their amides which are potential precursors of α-substituted butyrolactams and lactones. Since (1S,2R)-ephedrine is commercially available, the methodology also gives access to the enantiomeric series of α-hydroxy acids.