ChemInform Abstract: Revised Structure of Flavolipin and Synthesis of Its Isomers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
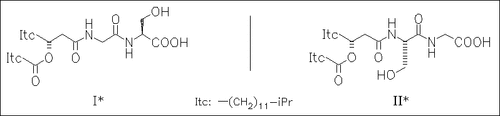
Detailed synthetic studies reveal that none of the diastereomers of type (II) corresponds to the structure of the serine-containing lipid flavolipin. However, the spectral data are consistent with (I) and its antipode. The macrophage stimulation of (I) corresponds to that of the natural compound, whereas the enantiomer is found to be inactive.