ChemInform Abstract: An Expeditious Route to the Synthesis of Highly Functionalized Chiral Oxepins from Monosaccharides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
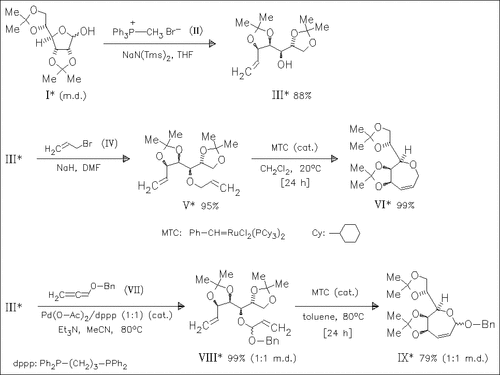
A novel and versatile route to highly functionalized chiral oxepins [cf. (VI) and (IX)] derived from partially protected aldofuranoses such as (I) is reported. The transformation proceeds via Wittig olefination of the anomeric center followed by allylation or oxopalladation of (III). Ring-closing metathesis of the resulting dienes (V) and (VIII) yields the ring-expanded products.