ChemInform Abstract: Coordination Ambidence of the Thiocyanate Anion in Alkali Trithiocyanatomanganate(II) Hydrates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The compounds NH4[Mn(NCS)3H2O] (I), K[Mn(NCS)3H2O] (II), and Na[Mn(NCS)3]×3H2O (III) are prepared from Mn(NCS)2 and thiocyanate in aqueous solutions. Single crystal XRD of (I) and (II) shows orthorhombic symmetry with space group Pbca and Z = 8. Compound (III) is of trigonal symmetry, space group P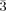 and Z = 2. (I) and (II) form polymeric layers which are characterized by the ambident nature of the thiocyanate ligand and binding of the water molecule to the octahedrally coordinated Mn atom. In (III) the complex manganate (II) anions form a layer structure with trigonal symmetry in which each Mn atom is connected to three Mn neighbors by thiocyanate double bridges.
and Z = 2. (I) and (II) form polymeric layers which are characterized by the ambident nature of the thiocyanate ligand and binding of the water molecule to the octahedrally coordinated Mn atom. In (III) the complex manganate (II) anions form a layer structure with trigonal symmetry in which each Mn atom is connected to three Mn neighbors by thiocyanate double bridges.




