ChemInform Abstract: Enantiospecific Generation of anti-Aldol Adducts via Conjugate Addition to 5-Methylene-1,3-dioxan-4-ones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
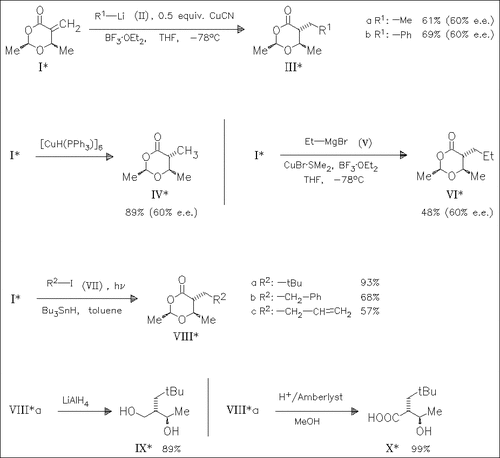
Michael addition reactions to the exocyclic olefinic double bond of the optically pure dioxane (I) are investigated. The reaction with cuprates, Stryker′s reagent or copper-mediated addition of Grignard reagents [cf. (V)] proceeds with poor diastereoselectivity. However, radical addition of alkyl iodides leads to the trans products (VIII) with complete diastereoselectivity. The adducts [cf. (VIIIa)] can be easily opened under reductive and acidic conditions.