ChemInform Abstract: Total Synthesis of (+)-3-Oxacarbacyclin. Part 2. Stereoselective Deprotonation and Completion of the Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
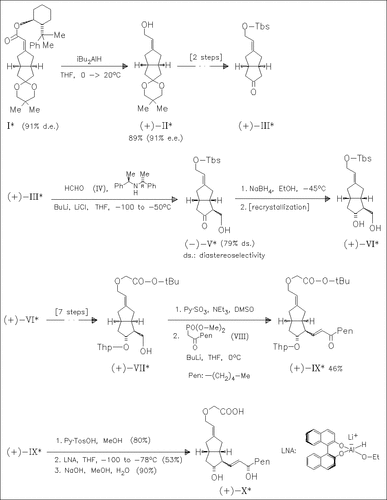
The key steps in the synthesis of the title compound (X) are diastereoselective formation of the ester (I) described in the preceding paper, stereoselective deprotonation of the ketone (III) and stereoselective reduction of the keto group in compound (IX). This method can also be applied to prepare cicaprost, eptaloprost, and other side-chain modified 3-oxacarbacyclins.