ChemInform Abstract: [3 + 2] Cycloaddition Reactions of Alkynyl(phenyl)iodonium Triflates with Ethyl Diazoacetate, N-t-Butyl-α-phenyl Nitrone and t-Butylnitrileoxide as 1,3-Dipoles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
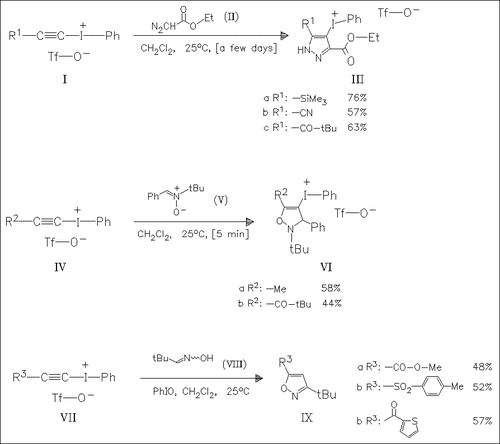
Iodonium triflates undergo 1,3-dipolar cycloadditions with ethyl diazoacetate (II), the nitrone (V) and tert-butylnitrile oxide generated in situ from oxime (VIII) and iodosobenzene. Each reaction yields only a single regioisomer. Displacement of the iodonium moiety in the heterocycles (III), (VI), and (IX) by nucleophiles allows the preparation of functionalized systems.