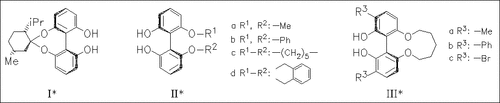
ChemInform Abstract: General Method for Asymmetric Synthesis of Substituted 2,2′-Biaryldiols via Asymmetric Desymmetrization of 2,2′,6,6′-Tetrahydroxybiphenyl with l-Menthone.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The asymmetric desymmetrization of prochiral 2,2′,6,6′-biphenyltetrol is effectively achieved by acetalization with l-menthone. The resulting isomenthonide (I) of S-axial chirality allows the preparation of a variety of 6,6′-dialkoxy- and aromatic substituted derivatives (II) and (III).