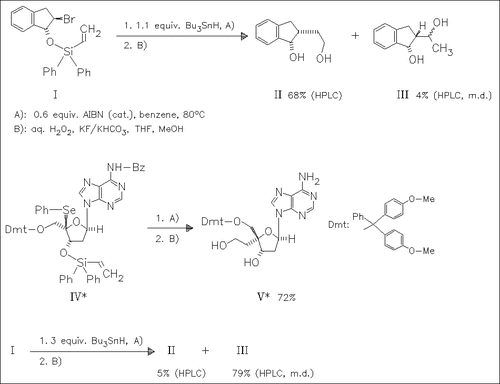
ChemInform Abstract: Nucleosides and Nucleotides. Part 165. A Novel Ring-Enlargement Reaction of (3-Oxa-2-silacyclopentyl)methyl Radicals into 4-Oxa-3-silacyclohexyl Radicals. Stereoselective Introduction of a Hydroxyethyl Group via Unusual 6-endo-Cyclization Products Derived from 3-Oxa-4-silahexenyl Radicals and Its Application to the Synthesis of a 4′-α-Branched Nucleoside.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The title radical reaction with a vinylsilyl tether is applicable to a variety of halohydrins and related compounds for introducing a hydroxyethyl group at the β-position of the hydroxyl with cis-configuration. Reaction conditions are optimized to get the desired products as major compounds.