ChemInform Abstract: Nitriles under Palladium-Catalyzed Hydrogenation Conditions as Substitutes for Aldehydes in the Reaction with 1,2-Amino Alcohols: Formation of 1,3-Oxazolidines and Reductive N-Alkylation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
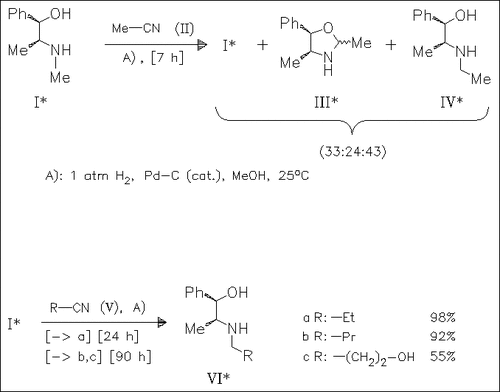
Nitriles [cf. (II)] react with (-)-ephedrine in the presence of hydrogen and catalytic amounts of Pd—C to form 1,3-oxazolidines (III) which are directly cleaved by reduction of the NC—O bond to the 1,2-amino alcohols (IV). Prolonged reaction times results in a selective one-pot synthesis of the N-alkylated amino alcohols such as (VI).