ChemInform Abstract: Synthesis and X-Ray Crystallographic Study of Cyclobis-(1→2)-α-D-glucopyranosyl Peracetate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
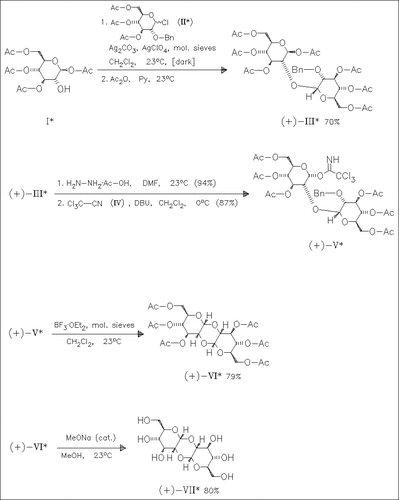
A facile synthesis of title compound (VI), which is easily transformed to cyclokojibiose (VII), is achieved in excellent yield. Key step is the novel activated anomeric carbon atom-mediated debenzylation of (V), which proceeds with complete selectivity and gives (VI) as the single product. X-ray crystallographic studies of (VI) reveal, that the glucopyranosyl moieties are interconnected by a 1,4-dioxane moiety, which is in a boat conformation with the anomeric carbon atoms in the bow positions.