ChemInform Abstract: Stereoselective Addition of 2-Furyllithium and 2-Thiazolyllithium to Sugar Nitrones. Synthesis of Carbon-Linked Glycoglycines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
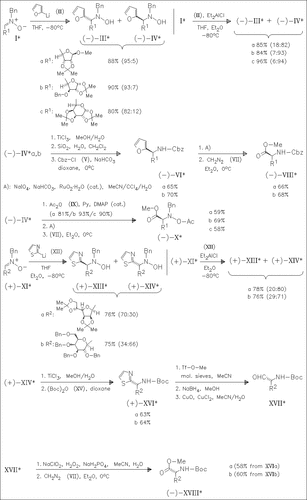
A new and quite general route to sugar glycines in both epimeric forms is reported based on stereoselective addition of the lithium compounds (II) and (XII) to the sugar nitrones (I) and (XI). Opposite diastereofacial selectivity is observed depending on the presence or absence of Et2AlCl.