ChemInform Abstract: Reactivity of Chiral Oxazaphospholidines on Activated Halide Compounds: Synthesis and Coordination Studies of Chiral Hybrid Phosphine—Phosphine Oxide Ligands.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
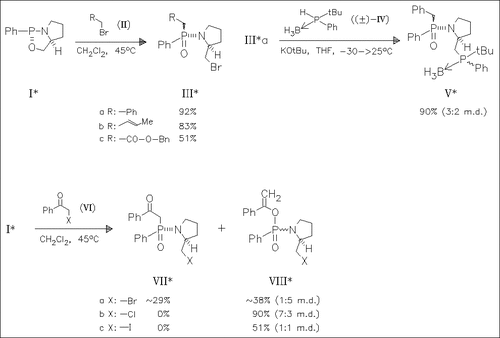
Treatment of the oxazaphospholidine (I) with alkyl bromides of type (II) leads to phosphinamides with total diastereoselectivity. The reaction with α-haloacetophenones gives ketophosphinamides and diastereomeric vinylphosphonamides. Starting from the phosphinamide (IIIa) chiral hybrid phosphine—phosphine oxide ligands like (V) are prepared. (RP)-Benzylphenyl [2-(S)-diphenylphosphinomethylpyrrolidin-1-yl]phosphine oxide is found to act generally as a monodentate ligand in neutral metal complexes and as a bidentate ligand in cationic metal complexes. It is also applicable to the synthesis of heterobimetallic complexes.