ChemInform Abstract: Ring Expansion of Isopropenylcyclopropanes to Dihydromethyloxepins.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
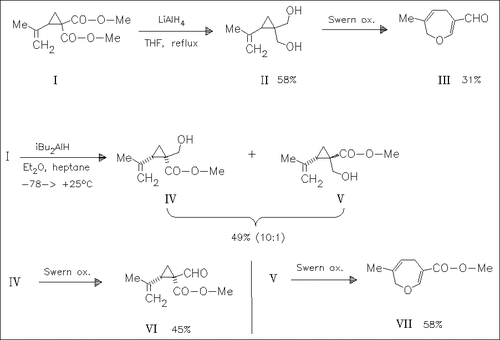
The stereochemistry is studied concerning the ring expansion of cyclopropane derivatives such as (I). LAH reduction of (I) followed by Swern oxidation leads to oxepin (III) via the corresponding diol and formyl compounds. The DIBAL reduction of (I) affords the isomeric esters (IV) and (V) and subsequent Swern oxidation of (V) causes a ring expansion giving oxepin (VII). However, Swern oxidation of ester (IV) causes no ring expansion. The ring expansion might need the cis-stereochemistry between the isopropenyl and the formyl group.