ChemInform Abstract: Organic Nitrates. Part 2. Synthesis and Biological Activities of 4-Nitrooxymethylphenyl-1,4-dihydropyridines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
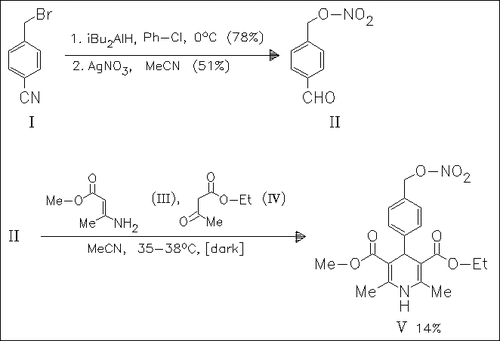
In order to obtain new vascular active substances synthetic procedures for 2-nitrooxymethyl-4-phenyl- and 4-nitrooxymethylphenyl-1,4-dihydropyridines, e.g. (V), are tested. The first ones are not available because of their spontaneous lactonization. The second type (V) is prepared by the Hantzsch reaction and found to be negative inotropic. The vasodilator activity of all derivatives is less than that of nitrendipine.