ChemInform Abstract: Imidazo[2,1-b]thiazolium Salts on the Basis of 2-Phenylamino-4-methylthiazole.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
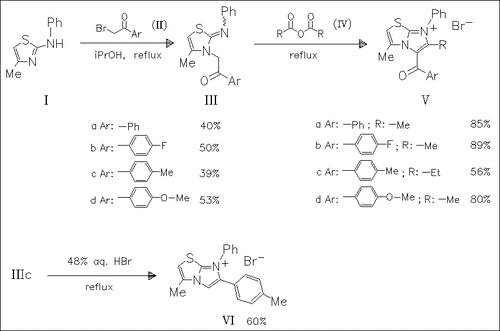
Reaction of the aminothiazole (I) with phenacyl bromides proceeds regioselectively at the endocyclic nitrogen atom to give the N-(phenacylmethyl)substituted thiazoles (III) which on the action of dehydrating agents can undergo cyclization. Thus, in the presence of hydrobromic or formic acid the 2-arylimidazothiazole (VI) is obtained while under the action of propionic or acetic anhydride (IV) the 2-alkyl-3-phenacylimidazothiazoles (V) are formed.