ChemInform Abstract: Alkylation of an Imidazolidine Enaminoester: A New Sequence for the Cα-Alkylation of 4,5-Dihydroimidazoles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
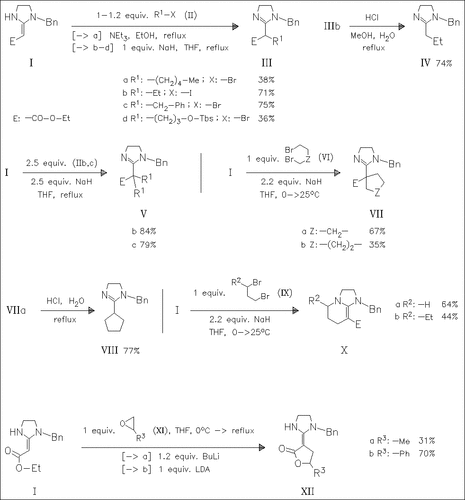
Basic treatment of the enaminoester (I) with halogenoalkanes and epoxides successfully leads to C-alkylated products. Subsequent removal of the ester group offers a new route to 2-alkylated 4,5-dihydroimidazoles. With 1,3-dihalogenoalkanes C,N-dialkylation takes place yielding imidazopyridines like (X) efficiently.