ChemInform Abstract: Dihydrobenzofuran Analogues of Hallucinogens. Part 4. Mescaline Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
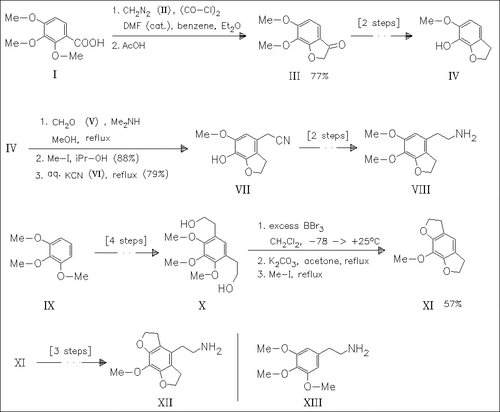
Elaboration of the aminoethyl side chain by standard sequence of electrophilic formylation, condensation with nitromethane and subsequent reduction of the nitrostyrene is unsuccessful in the synthesis of (VIII), but is used in the preparation of (XII). An alternative method to (VIII) starts with regioselective demethylation of the 7-methoxy group to give (IV) and leads to target compound (VIII) in good overall yield. In comparison with hallucinogen mescaline (XIII), its rigid analogues (VIII) and (XII) possess increased overall hydrophobicity and enhanced affinity for serotonin 5-HT2 receptors, but decreased efficacy and less hallucinogenic activity.