ChemInform Abstract: Palladium-Catalyzed Allylic Substitution in γ-Oxygenated Vinyl Sulfones: One-Step Synthesis of Tetrasubstituted Dihydrofurans.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
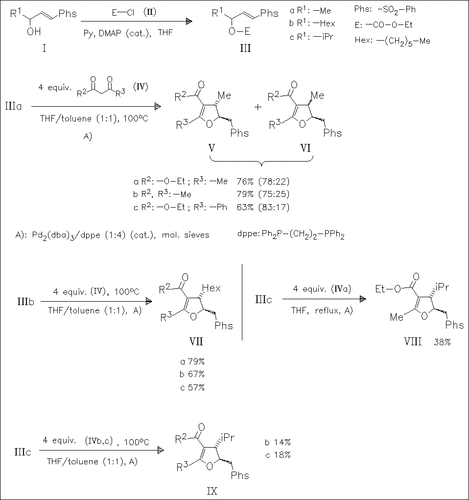
A convergent and stereoselective one-step synthesis of highly substituted dihydrofurans by Pd(0)-catalyzed reaction of the carbonates of γ-hydroxy vinyl sulfones (III) with β-ketoesters and 1,3-diketones is described. Since vinylsulfones (I) can be readily prepared in enantiomerically pure form, this method should provide a general new access to enantiopure tetrasubstituted derivatives.