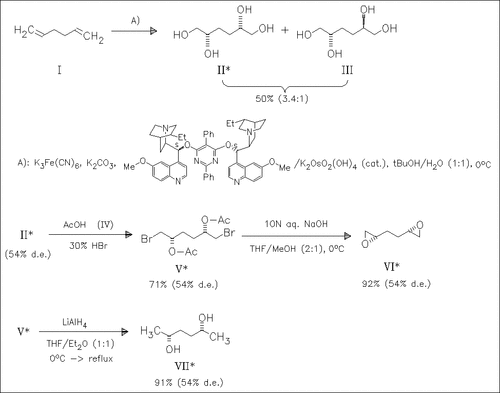
ChemInform Abstract: Double Asymmetric Dihydroxylation of 1,5-Hexadiene.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The title reaction gives in one-step a mixture of the tetrols (II) and (III) in a ratio of 3.4:1. From this ratio a facial selectivity of 6.65:1 for each double bond can be calculated. The compounds (II)/(III) serve as starting material for the preparation of the derivatives (VI) and (VII). While the separation of the diastereomers is not possible so far, this route for their preparation is useful because it is very short.