ChemInform Abstract: Highly Enantiofacial Protonation of Prochiral Lithium Enolates with Chiral β-Hydroxy Sulfoxides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
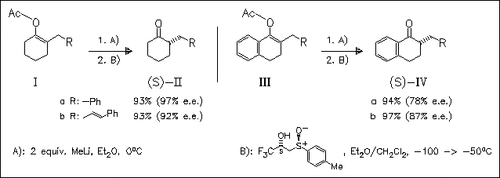
Treatment of 2-alkylated enol acetates such as (I) and (III) with MeLi gives prochiral lithium enolates. They undergo an enantiofacial protonation using chiral β-hydroxy sulfoxides. Best results are obtained under the optimized conditions shown which provide 2- substituted cyclohexanone derivatives (II) and (IV) with excellent e.e.