ChemInform Abstract: Reaction of Lactim Ethers and Lactim Sulfides with Electrophiles: Attack at Nitrogen Followed by Ring-Opening under Neutral Conditions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
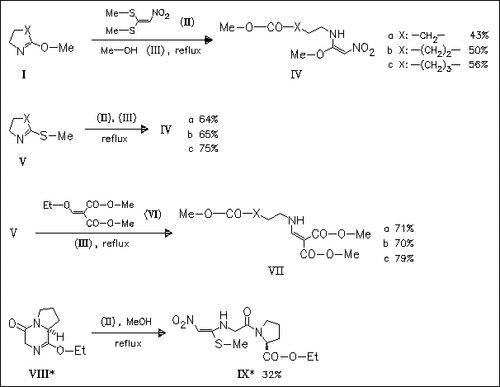
Title compounds (I) and (V) are attacked exclusively at the ring nitrogen atom when treated with electrophilic push-pull ethylenes. Substitution is followed by a ring opening reaction. Lactim sulfides give higher yields than the corresponding lactim ethers. The new route also provides useful intermediates for the synthesis of peptides incorporating non-natural α-alkylated amino acids (→ (IX)) .