ChemInform Abstract: Dinitrogen Tetroxide Complexes of Iron and Copper Nitrates as New Reagents for Selective Mono- and Dinitration of Phenolic Compounds.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
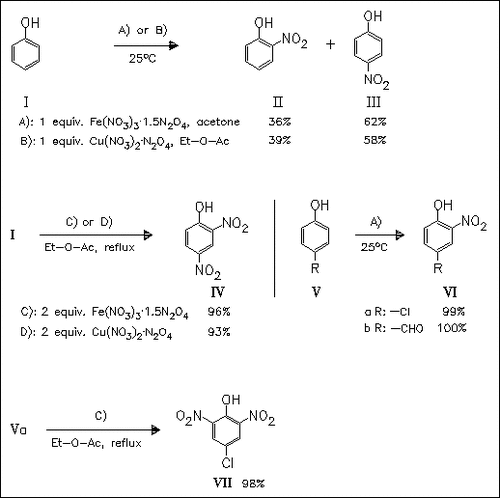
Solid complexes of Fe(NO3)3 and Cu(NO3)2 with gaseous N2O4 prove to be efficient agents for the nitration of phenol and 4-substituted phenols. In comparison with other metal nitrates high rate, yield, and regioselectivity enhancements are observed. The selectivity for the formation of mono- or dinitration products can be easily controlled by changing the nature of the solvent and reaction temperature.