ChemInform Abstract: Chemical Transformation of 1,8-Cineole. Synthesis of N-Phenylimides from Cineolic Acid.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
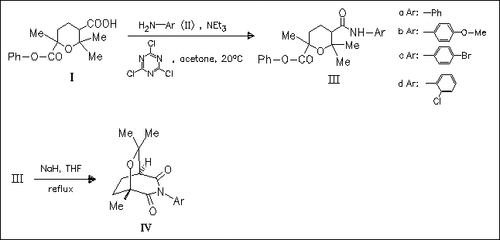
Cineolic acid monophenyl ester (I) is regioselectively converted into the corresponding N-arylimides (IV) in two steps. The regioselectivity of some of the reactions and the heterocyclic ring conformations are established based on spectroscopic data. Imides (IV) are unstable in contact with moisture and undergo regioselective hydrolysis.