ChemInform Abstract: Remote Stereochemical Control of Both Reacting Centers in Ketyl-Olefin Radical Cyclizations: Involvement of a Samarium Tridentate Ligate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
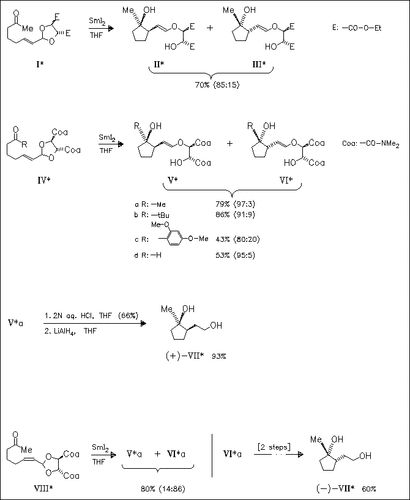
The SmI2-mediated cyclization of tartrate-derived keto allylic acetals provides the first example of an asymmetric radical cyclization in which high levels of stereochemical induction are achieved at both reacting centers. This transformation also demonstrates the first example of the use of a chelating metal to effect high levels of remote asymmetric induction in a radical reaction (mechanism). The stereochemistry of the major isomer (Va) is established by an X-ray analysis. Cyclization of the Z-isomer (VIII) proceeds with the same high degree of relative stereoselectivity. Through this novel method the preparation of enantiomerically enriched cyclopentanediols can be achieved as demonstrated for the enantiomers (VII).