ChemInform Abstract: Enantioselective Synthesis of the C11-C24 Segment of Macrolactin A via Organoiron Methodology.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
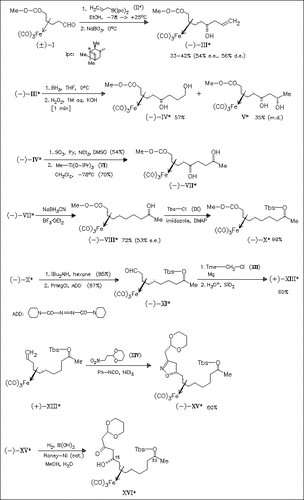
The C11-C24 segment (XVI) of the antiviral active polyene macrolide macrolactin A is synthesized in optically active form starting from iron-complexed racemic dienoate (I). The (diene)Fe(CO)3 functionality controls both the C23 stereochemistry via a chirality relay strategy and the C15 stereocenter via a highly diastereoselective nitrile oxide- olefin cycloaddition.