ChemInform Abstract: Reductive Cyclization of 6-Cyanomethyl-5-nitropyrimidines - An Efficient Route to 7-Alkyl-5H-pyrrolo(3,2-d)pyrimidines and 6-Amino-7, 7-dialkyl-7H-pyrrolo(3,2-d)pyrimidines. Utilization in the Synthesis of 9-Deaza Analogues of DHPA.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
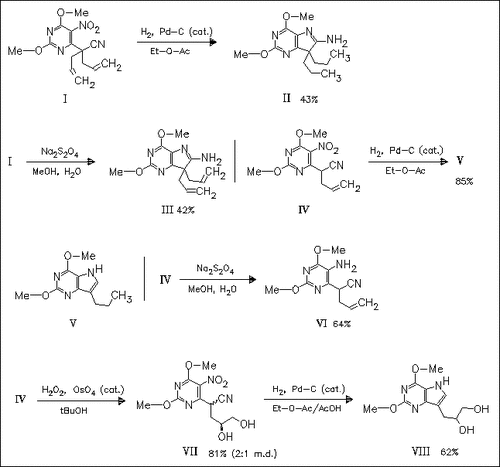
In the presence of Pd-C the nitropyrimidines (I) and (IV) undergo reductive cyclization to the 7-alkylpyrrolopyrimidines (II) and (V). The reaction with Na2S2O4 gives the intermediary derivatives (III) and (VI), respectively. The reductive cyclization can also be applied to prepare the pyrrolopyrimidine (VIII), an intermediate for the synthesis of 9-deaza analogues of DHPA. In this case, addition of AcOH is necessary for successful reaction.