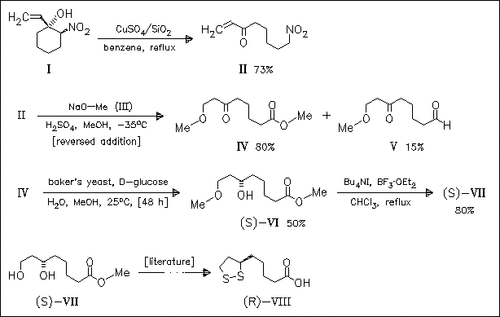
ChemInform Abstract: A Short Enantioselective Formal Synthesis of Methyl (S)-(-)-6,8- Dihydroxyoctanoate: A Key Intermediate for the Synthesis of (R)-(+)-. alpha.-Lipoic Acid.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The enantioselective synthesis of ester (VII), a known intermediate in the total synthesis of the title acid (VIII), uses a novel retro-Henry reaction of the ω-nitro α,β-unsaturated ketone (II) and the kinetic enzymatic resolution of the oxo ester (IV) as key steps.