ChemInform Abstract: Stereoselective Synthesis of Petrosterol and a Formal Synthesis of Aragusterols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
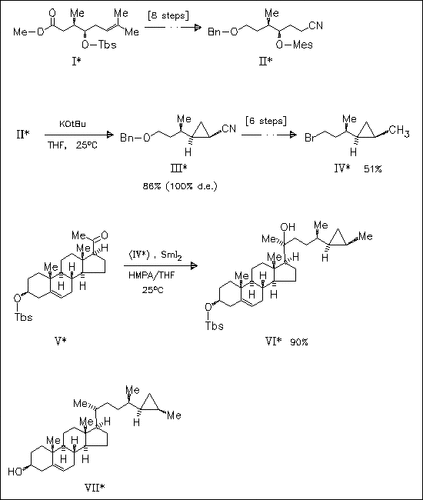
The synthesis of petrosterol (VII) is achieved via a stereoselective approach to the key intermediate (III). The procedure developed may be of more general interest for synthesizing steroids with cyclopropanes in their side chains.