ChemInform Abstract: Synthesis of a Tetrahydropyrano(2,3-d)oxazole Analogue of Trehazolin.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
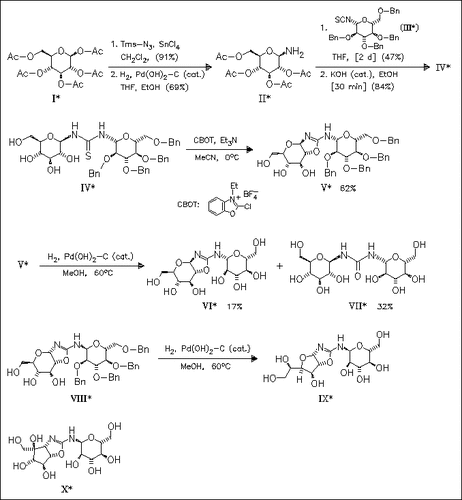
The title compound (VI) is synthesized as an analogue of the glycosidase inhibitor trehazolin (X) starting from the bis(β-D- glucopyranosyl)thiourea (IV). In contrast, the same reaction sequence starting from the corresponding bis(α-D-glucopyranosyl)thiourea leads to the furo(2,3-d)oxazole (IX). Both substances are inactive toward various glycosidases.