ChemInform Abstract: Immunosuppressive Cyclolignans.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
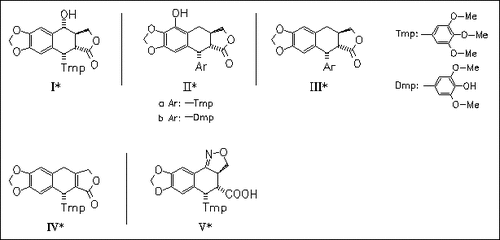
Derivatives (IV), (V), and, especially, (IIIb) are the most potent in vitro immunosuppressive compounds of a series of cyclolignans, e.g. (I) -(V), either isolated from Podophyllum resin, e.g. (I) and (II), or synthesized from (I) or (IIIa). Due to their better noncytotoxic activity, compounds (IV) and (V) are candidates for further in vivo evaluation.