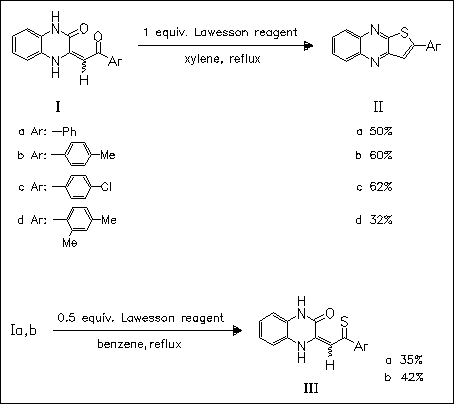
ChemInform Abstract: Reaction of 3-Aroylmethylene-1,2,3,4-tetrahydroquinoxalin-2-ones with Lawesson′s Reagent and Biological Activity of the Reaction Products.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The reaction of the title aroylmethylene-quinoxalinone derivatives (I) with Lawesson′s reagent provides the corresponding arylthiocarbonyl derivatives (III) or 2-arylthiophenoquinoxalines (II) depending on the amount of the sulfuration reagent employed. While the first mentioned quinoxalines (III) show only low activity against St. aureus and E. coli strains, the cyclized products (II) show enhanced antimicrobial activity.