ChemInform Abstract: Stereoselective Synthesis of Differentially Protected Derivatives of the Higher Amino Sugars Destomic Acid and Lincosamine from Serine and Threonine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
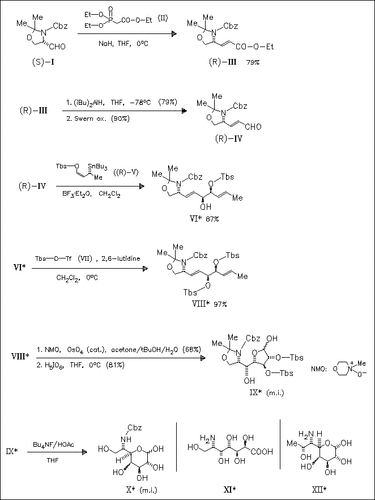
The pyranose (X), a precursor of destomic acid (XI), the aminoheptose component of destomycin and hygromycin antibiotics, is synthesized starting from the oxazolidine derivative (I) of N- benzyloxycarbonylserinal. An analogous reaction sequence starting from N-t-butoxycarbonylthreoninal leads to lincosamine (XII), the aminooctose component of the antibiotic lincomycin.