ChemInform Abstract: 3- and 4-Substituted 4H-Pyrido(4,3-e)-1,2,4-thiadiazine 1,1-Dioxides as Potassium Channel Openers (PCO): Synthesis, Pharmacological Evaluation, and Structure-Activity Relationships.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
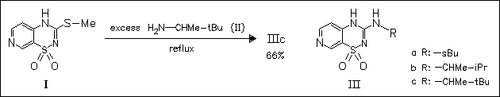
4-N-Substituted and -unsubstituted 3-alkyl- and 3-(alkylamino)-4H- pyrido(4,3-e)-1,2,4-thiadiazine 1,1-dioxides are prepared and tested against diazoxide and selected 3-alkyl- and 3-(alkylamino)-7-chloro-4H- 1,2,4-benzothiadiazine 1,1-dioxides as PCO on pancreatic and vascular tissues. The derivatives (III) appear to be powerful inhibitors of insulin release and are clearly more selective than their chlorobenzenic homologues or than diazoxide for pancreatic tissue versus the vascular tissue. Pyridothiadiazine PCO may serve as pharmacological tool in studying the KATP channel in numerous tissues.