ChemInform Abstract: Synthesis and Stereochemistry of the Epoxides of 2-Arylmethylidene-1- tetralones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
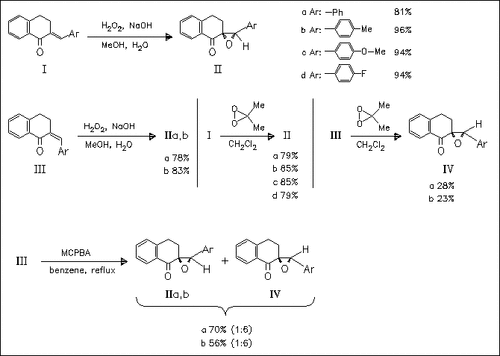
The use of hydrogen peroxide as oxygen source in the epoxidation of the trans and cis configurated alkenes (I) and (III) affords the corresponding trans-epoxides (II) as the sole products. In contrast, the use of dimethyldioxirane produces selectively the trans- or cis- epoxides (II) or (IV) in dependence on the stereochemistry of the starting material. This is the only method for the stereoselective synthesis of cis-epoxides. With MCPBA, cis-trans mixtures of epoxides are obtained.