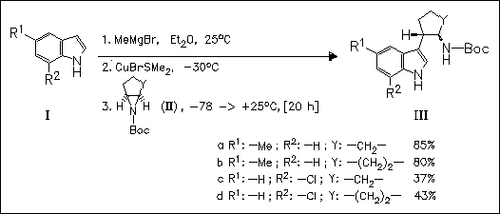
ChemInform Abstract: Conformationally Constrained Serotonin Analogues: Stereoselective Synthesis of trans-3-(2-Aminocycloalkyl)indoles by Aziridine Ring Opening.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Trans-3-(2-aminocycloalkyl)indoles (III) are stereoselectively prepared by the nucleophilic ring opening reaction of the cycloalkylaziridines (II) and lower order magnesium cuprates generated from the corresponding indoles (I).