ChemInform Abstract: Selective Rhodium-Catalyzed Insertion of Carbon Monoxide into the Nitrogen-Oxygen Bond of Isoxazolidines. New Reduction, Migration, and Rearrangement Reactions Catalyzed by Iridium Complexes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
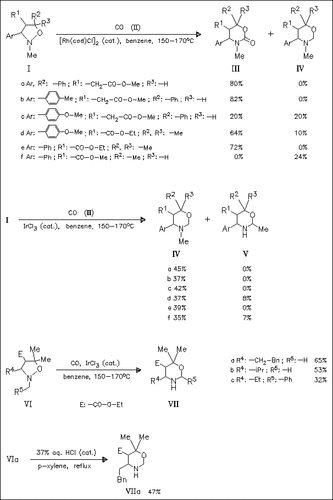
Rh-catalyzed reaction of isoxazolidines with carbon monoxide is found to afford 1,3-oxazin-2-ones of type (III) regioselectively and often in good yields. In the presence of iridium catalysts (IrCl3, Ir(CO)3Cl, (Ir(cod)Cl)2) a novel transformation of 3-arylisoxazolidines into tetrahydro-1,3-oxazines occurs via an intermolecular hydrogen transfer reaction. In some cases isomeric 1,3-oxazines of type (V) are also obtained. They are formed by a novel and unprecedented migration of the methyl group from nitrogen to the carbon atom arising from CO insertion. Finally, another unique rearrangement is observed in the Ir- catalyzed reaction of 3-alkyl substituted isoxazolidines (VI). Here, the substituents at nitrogen migrate into the ring yielding 1,3- oxazines like (VII). This rearrangement also takes place in the presence of HCl.